If you’re a stylist that regularly works with clients that have alopecia, we’ve got some good news: there’s a new drug that’s showing strong promise for treating the disease.
Concert Pharmaceuticals, a U.S.-based drug company, recently announced the completion of a Phase 3 clinical trial for a new, twice daily experimental pill that can combat hair loss. According to the company, this new drug—known as CTP-543—has been extremely successful in clinical trials, restoring almost a full head of hair in roughly 30-40 percent of people with alopecia.
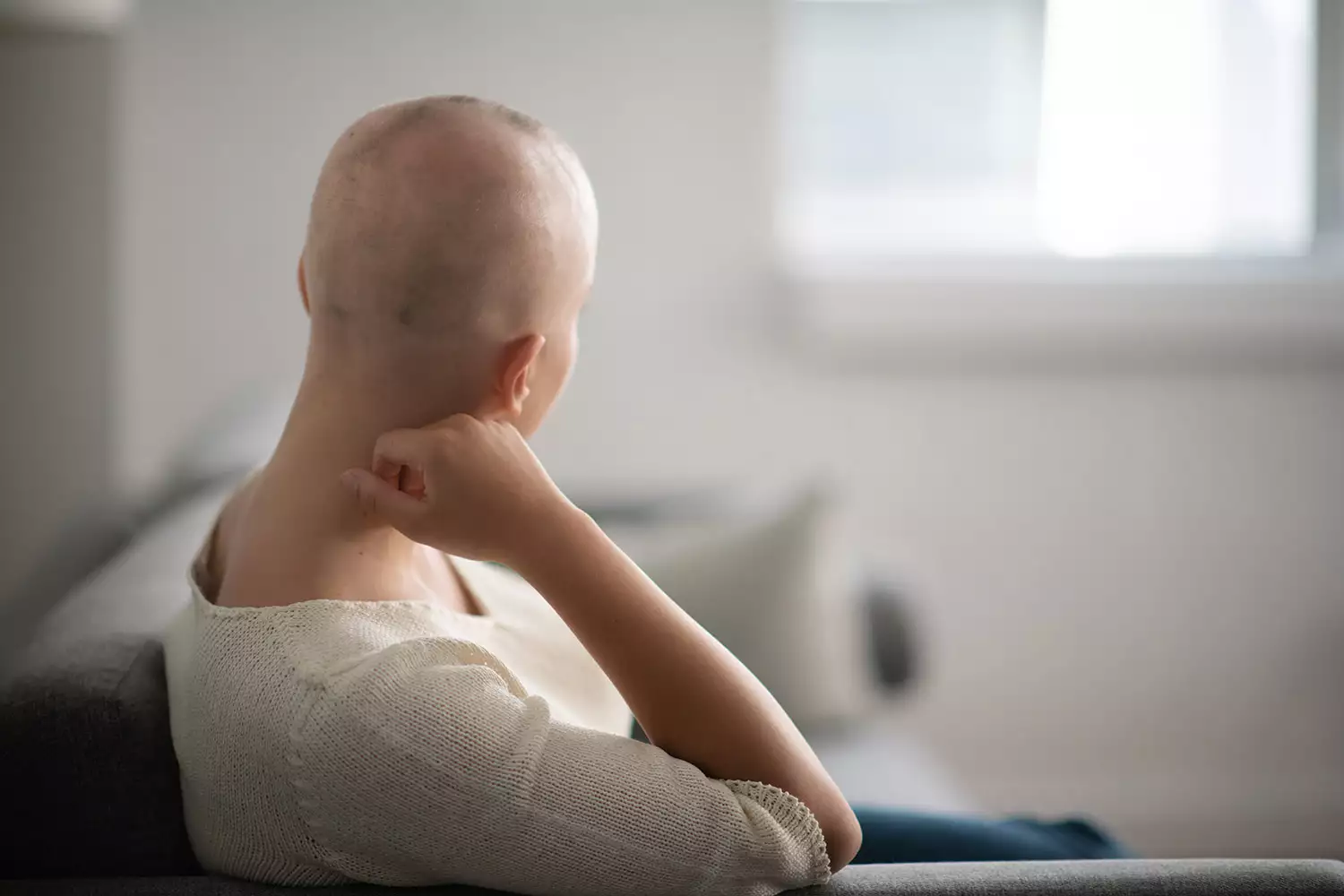
As part of the clinical trial, Concert tested their pill on a group of 706 people with varying degrees of hair loss (spanning moderate to severe) due to alopecia. The 24 week study explored how many people were able to regrow at least 80 percent of their hair. The company reports that 29.6 percent of people who received a medium dose of the drug and 41.5 percent of people who received a high dose of the drug successfully met this goal. Better yet, the drug was “generally well-tolerated” as less than 5 percent of patients complained of headaches, acne, and infections.
In case you didn’t already know, alopecia is an autoimmune disorder in which the immune system attacks the hair follicles, resulting in partial or complete hair loss. Currently, 6.8 million people in the U.S. suffer from alopecia—a group that also includes famous faces such as Rep. Ayanna Pressley, Viola Davis, and, most notably, Jada Pinkett Smith.
At the moment, there is no cure for alopecia, but Concert’s new drug could potentially help change that fact.
“Today marks an important milestone in advancing new treatments for alopecia areata, and I’m so happy to see such positive results from the first Phase 3 trial with CTP-543,” said Brett King, M.D., Department of Dermatology, Yale University School of Medicine and clinical investigator of THRIVE-AA1. “There is a great need for treatments for this challenging disease, and the results from the THRIVE-AA1 trial suggest that CTP-543 may potentially provide an important therapy for treating alopecia areata.”

Concert now hopes to repeat the Phase 3 trial again on more patients before presenting their findings and applying for U.S. Food and Drug Administration approval in 2023.
“With these compelling Phase 3 data, we believe that CTP-543 has the potential to be a best-in-class treatment for patients with alopecia areata, a disease that has long been ignored. We are extremely grateful to the patients and teams of clinical research professionals who participate in our trials,” said James V.Cassella, Ph.D., Chief Development Officer of Concert Pharmaceuticals. “We’re working to change the treatment landscape and hope that CTP-543 will be one of the first FDA-approved treatment options for this serious disease.”
For more information about Concert Pharmaceuticals, be sure to visit their website here.


